
Research and publications
Pep2Dia® Pre-Clinical and Clinical studies
Because a bioactive ingredient is an ingredient of food origin that has a natural action on health and is not a simple food (without being a drug), its manufacturing process is based on a set of steps, including several test phases whose role is to validate its effectiveness and the absence of side effects.
>> Pep2Dia® Pre-Clinical studies
Once the active peptide is identified, AP in the case of Pep2Dia®, the first validation step is completed during in vitro laboratory testing. Once the results were conclusive, the product obtained was produced in a ‘pilot version’. It was only when the search for the active dose was validated that clinical trials were established in 2019 before the final product launch.
>> Pep2Dia® Clinical studies
The clinical study was conducted to ascertain the effectiveness of the bioactive in people with prediabetes and identify the effective dose for humans.
To achieve this, the clinical study was conducted in two phases with people with prediabetes.
The 21 prediabetic volunteers, men and women, were recruited according to following selection criteria:
Aged between 30 and 70, the average age of participants in the study was 62
A fasting blood sugar level of between 1 and 1.25 g/l and/or glycated haemoglobin with an HbA1c level between 5.7 and 6.4% (fasting blood sugar is an instantaneous measure of the glycaemic state, while the HbA1c blood test assesses the glycaemic balance over a period of about two to three months)
A body mass index (BMI) of 19 to 35 kg/m²
Non-smokers
Caucasian
No change in eating habits or physical activity in the three months before screening and during the study
An absence of exclusionary causes (taking certain medications, certain diseases etc.)
The clinical trial was conducted in two phases.
The first phase was a double-blind randomized crossover study (randomized test product, with neither the patient, researcher nor doctor aware of which product has been administered).
In the trial, the product was given to volunteers 15 minutes before a high-sugar breakfast.
In the second stage of the clinical study, a supplement of 1,400 mg of Pep2Dia® was given for six weeks, before the main meal of the day.
The results of Pre-Clinical and Clinical Pep2Dia® studies
The preclinical and clinical Pep2Dia® studies have demonstrated a significant reduction in postprandial glycaemia, i.e., reduction in the blood’s glucose content after meals, and without affecting an increase in the secretion of insulin.
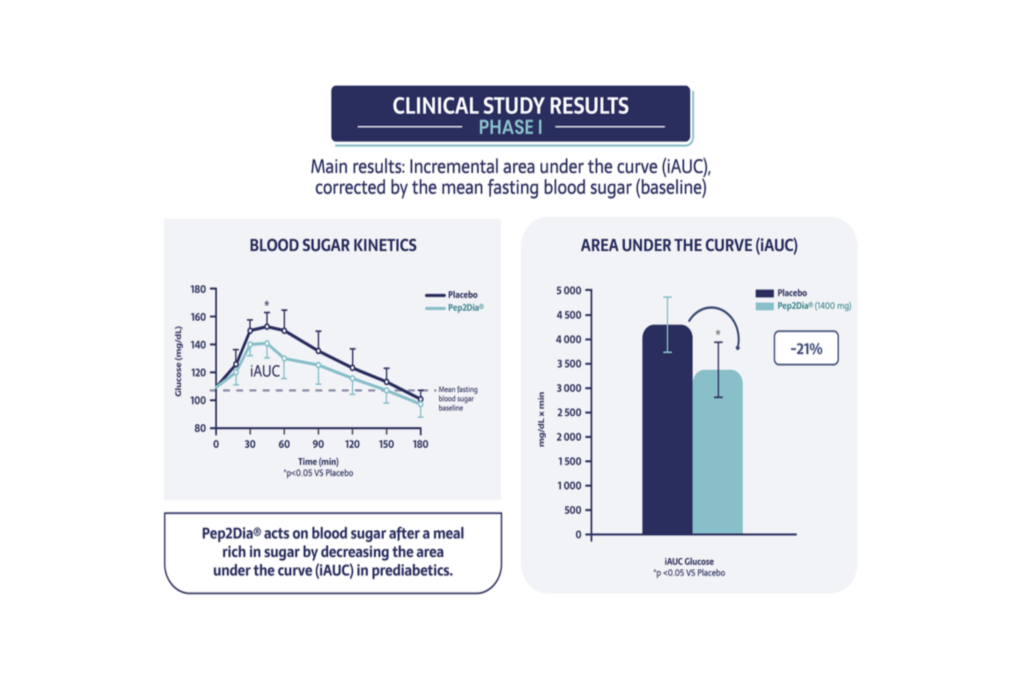
Clinical study results - Phase I
In fact, Pep2Dia® reduces blood sugar levels by 21% after a sweet meal in people with prediabetes.
Pep2Dia® also works by lowering the maximum blood sugar level after a meal high in sugar (-7% on the peak in blood sugar and -16% on the maximum increase in glucose compared to a placebo). In addition, Pep2Dia® does not increase insulin levels, which is important for people with prediabetes who have a tendency for hypersecretion of insulin.
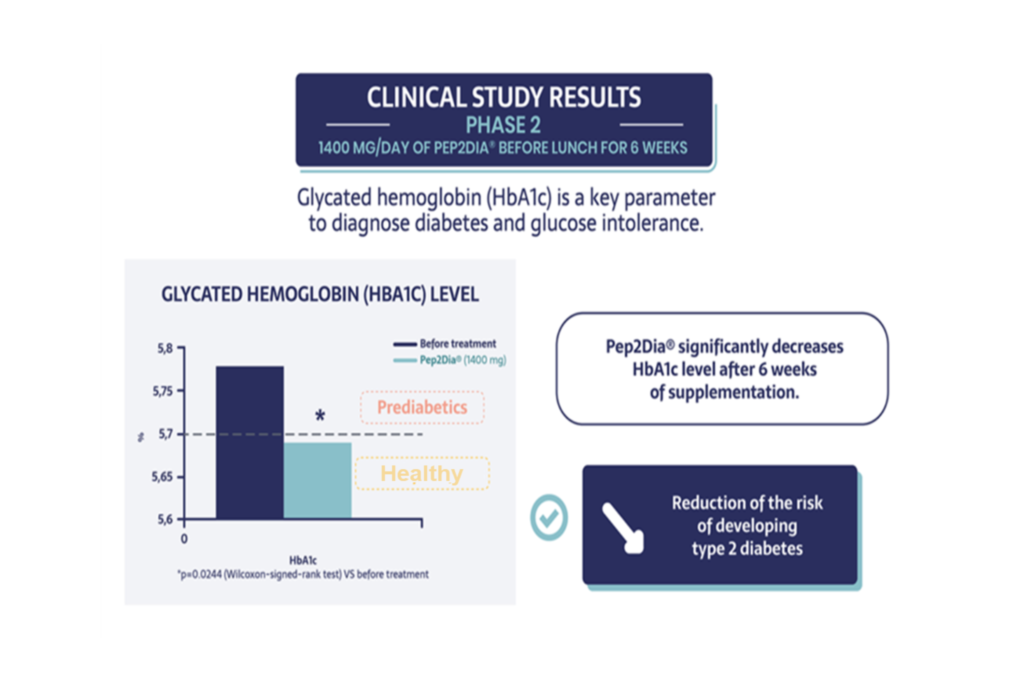
Clinical study results - Phase II
Finally, the results show that Pep2Dia® significantly decreases the level of glycated haemoglobin (HbA1c which characterises the history of blood sugar levels over the past three months), the main factor in detecting hyperglycaemia and the main symptom of prediabetes, after six weeks of supplementation.
In summary, bioactive Pep2Dia® acts on the main factors inducing diabetes and helps prediabetics to improve their health.
publications
Journal – Nutrients
Year – 2019
Journal – Current Development in Nutrition
Year – 2021

